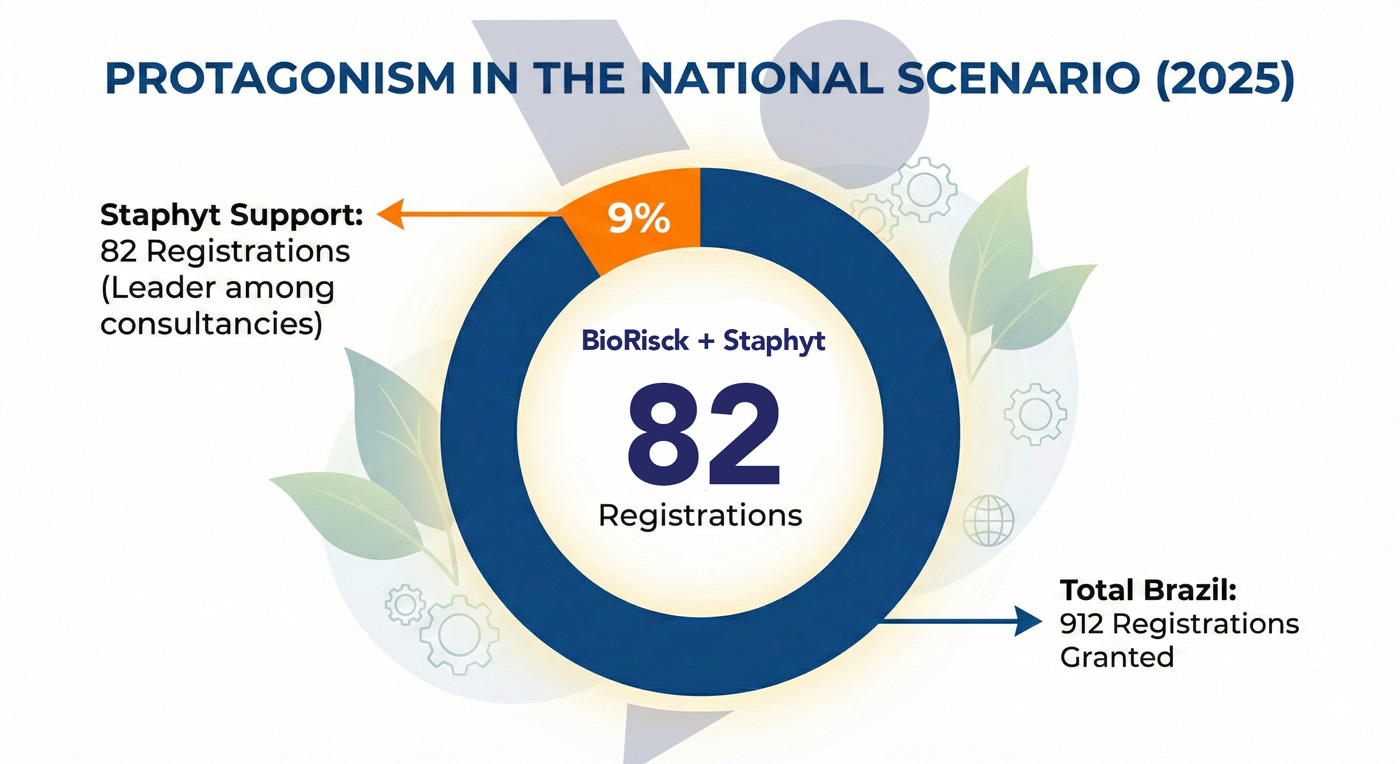
Staphyt once again leads product registrations in Brazil and consolidates four consecutive years of leadership in regulatory affairs

Regulatory affairs in Brazil: a context that amplifies the significance of the results
Regulatory affairs in Brazil involve complex processes conducted by multiple competent authorities, with detailed technical assessments, extensive documentation requirements, and long timelines. Methodological flaws, technical inconsistencies, or poorly defined strategies often result in additional requests and significant delays in product registrations.
In this context, maintaining leadership in registrations for four consecutive years is not the result of occasional volume, but rather of a mature operational model based on structured processes, deep regulatory knowledge, and accumulated practical experience.
Since entering the Brazilian market in 2020, Staphyt has applied the same technical and regulatory standards used in highly demanding markets such as the European Union, ensuring regulatory alignment, high-quality documentation, and greater predictability in regulatory processes.
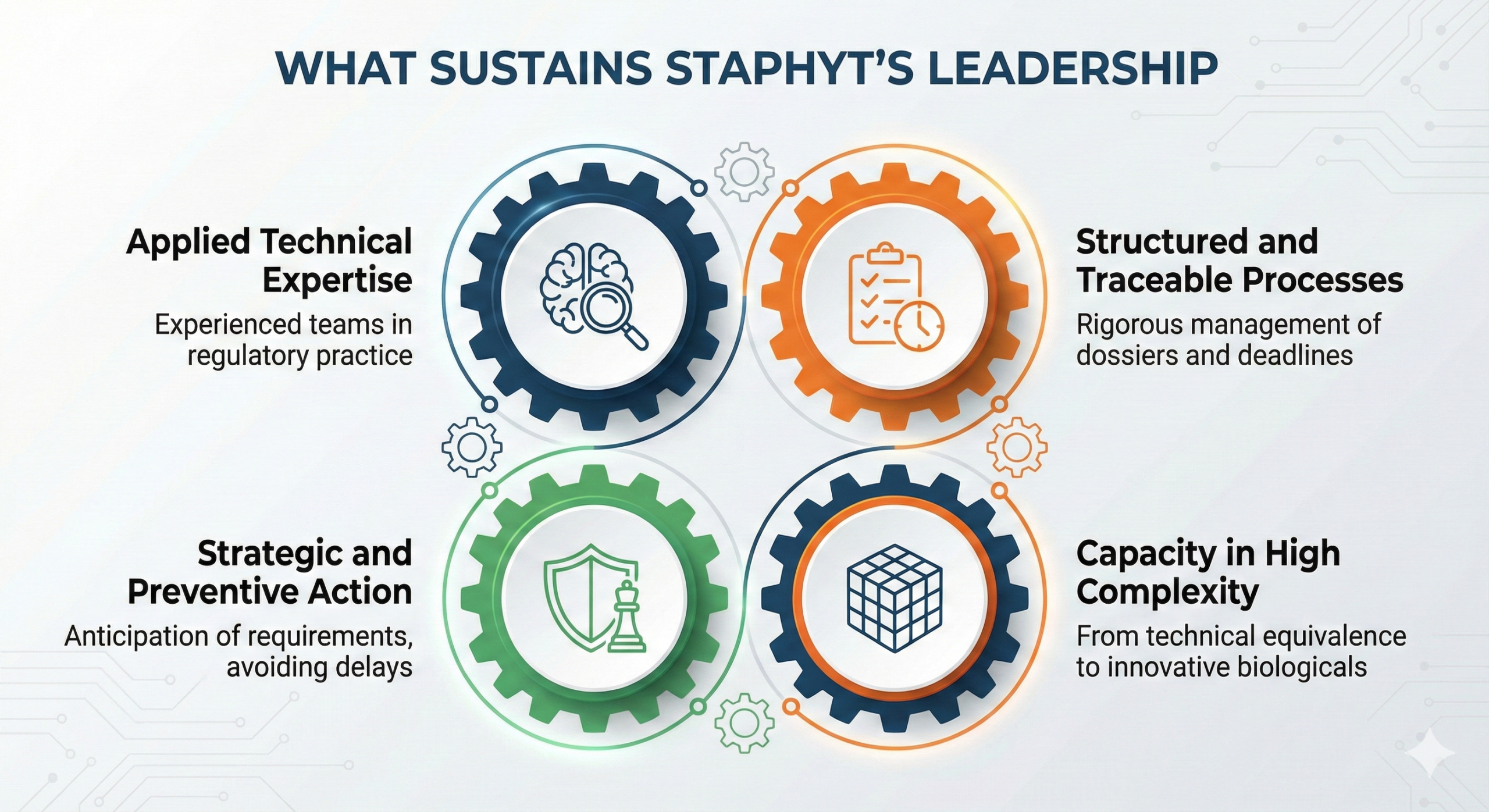
What sustains Staphyt’s leadership in regulatory affairs
Several factors explain the consistency of Staphyt’s results in product registrations in Brazil:
Applied technical specialization
Teams composed of analysts, coordinators, and managers with hands-on experience in regulatory affairs, legislation, and registration processes.
Structured and traceable regulatory processes
Rigorous management of dossiers, deadlines, and interactions with regulatory authorities, reducing risks and rework.
Strategic and preventive approach
Anticipation of regulatory requirements, avoiding reactive approaches that compromise timelines and technical decisions.
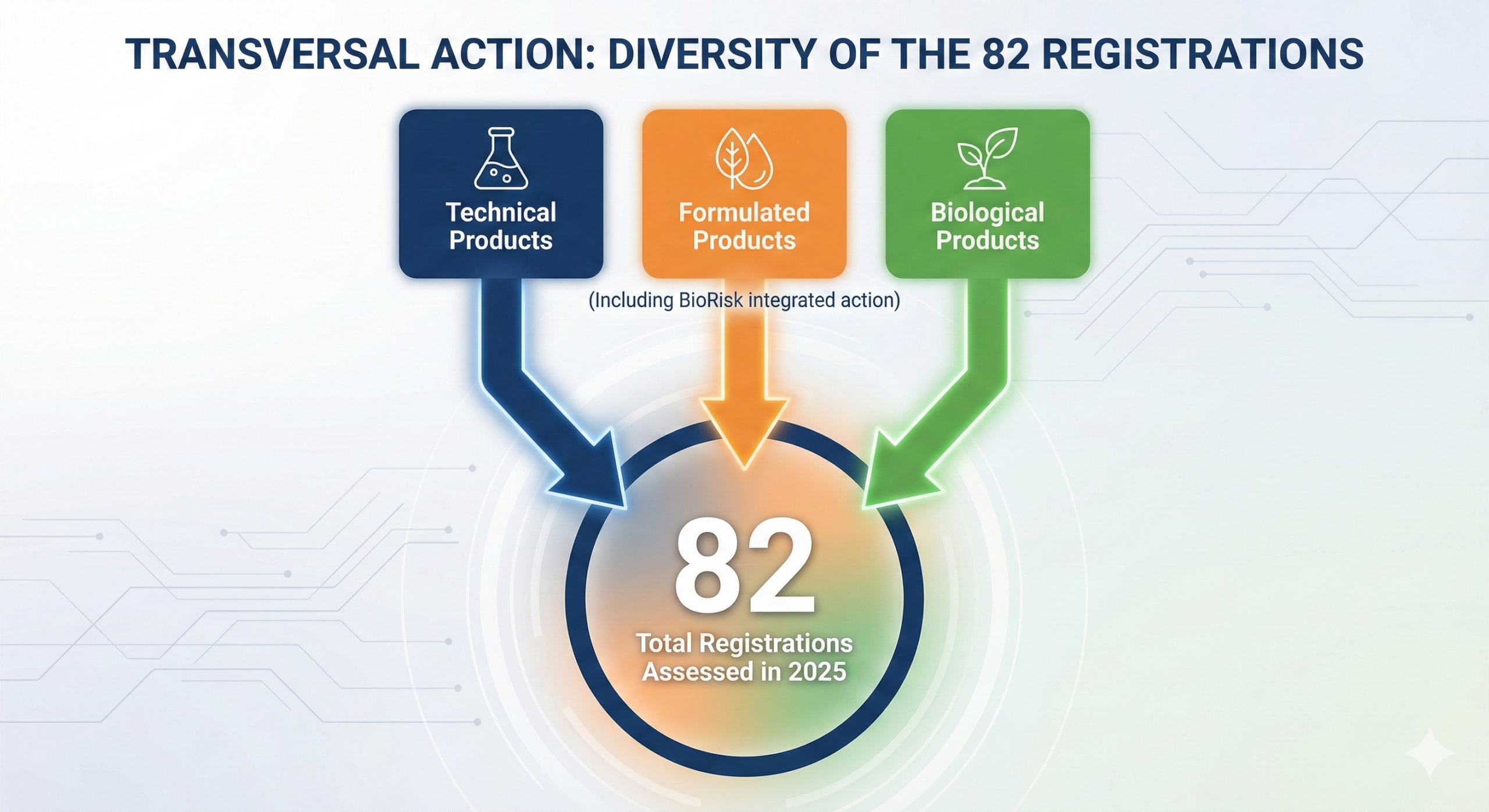
Ability to handle high regulatory complexity
From technical equivalence to biological product registrations and innovative formulations.
Know more about Regulatory Affairs in Brazil
As part of its commitment to disseminating technical knowledge in regulatory affairs, Staphyt will host an exclusive webinar with its Director of Regulatory Affairs (Brazil), Daniela Maia, on February 3rd at 3:00 p.m. CET.
Titled “Bioinputs Registration in Brazil: Current Regulatory Framework and Upcoming New Developments,” the webinar will address the current regulatory framework applicable to agricultural biological products, including different regulatory categories, authorization requirements, registration, and compliance. The roles of competent authorities, the main challenges faced by the sector, and a medium-term analysis of expected developments with the implementation of the new Bioinputs Law will also be discussed.
Participation is free of charge, upon prior registration:
https://staphyt.clickmeeting.com/bioinputs-registration-in-brazil-current-regulatory-framework-and-upcoming-new-developments/register
Sign up now!






